MCM CulLymT-SFM12(A) is a serum-free cell culture medium specialized for human T lymphocyte culture in large scale. MCM CulLymT-SFM12(A) is component clear, animal origin-free and low protein level. It can be applied in in vitro T lymphocyte personalized or large-scale culture without serum under diffrent kinds of culture model, including static culture, fed-batch and perfusion in SUB.
Liquid medium
Tobitec™ MCM CulLymT-SFM12(A) medium can provide various specifications of culture medium Liquid-Bottle, with the same performance and formula as liquid medium. Tobitec™ MCM CulLymT-SFM12(A) liquid medium is produced without animal origin, Tobitec™MCM CulLymT-SFM12(A) culture medium has the same performance and serum-free.
Product Information
Component clear, serum-free, animal origin-free and low protein level
Completely support serum-free culture, no serum or serum substitute required,and suitable for bioreactor
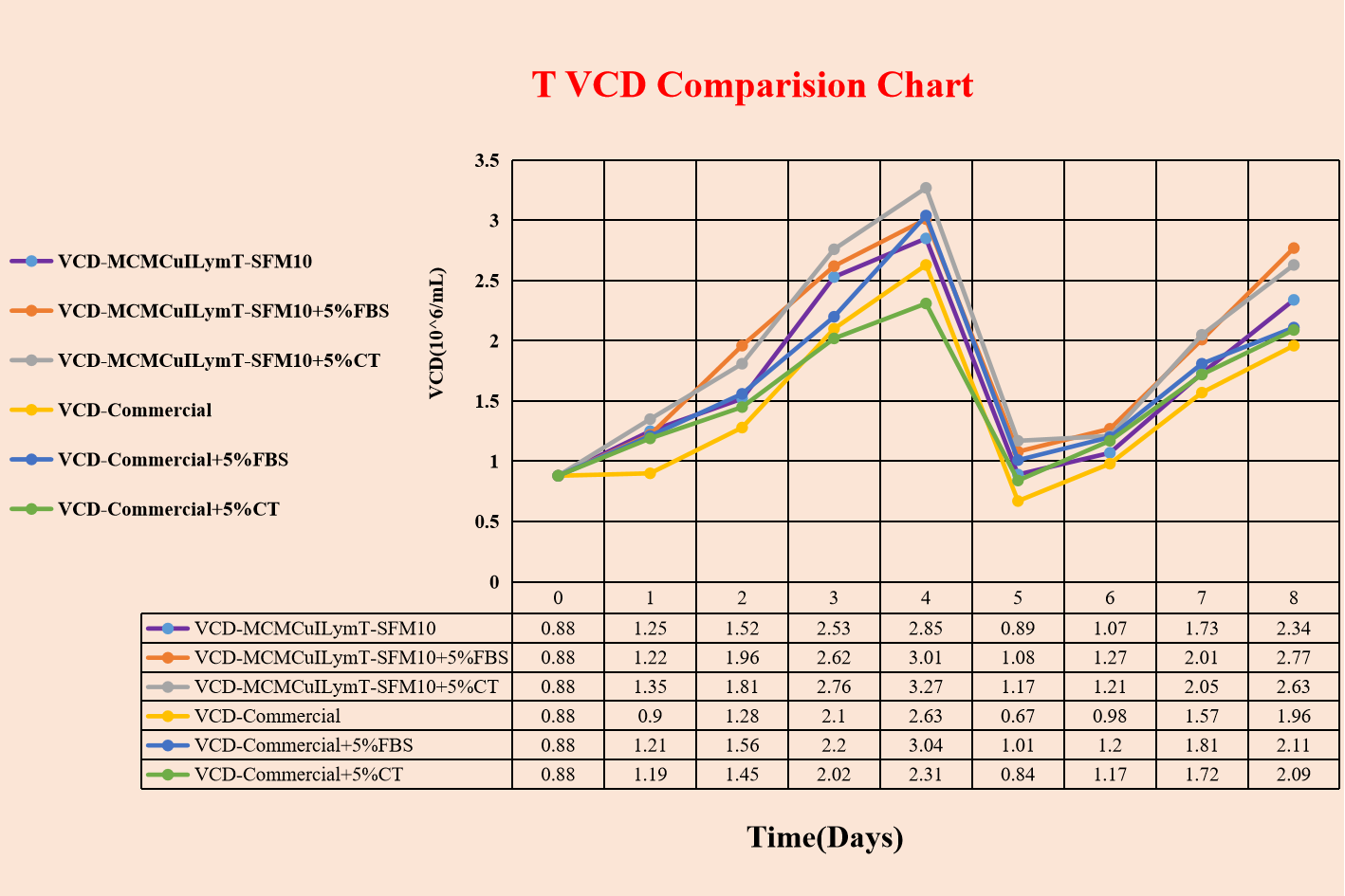
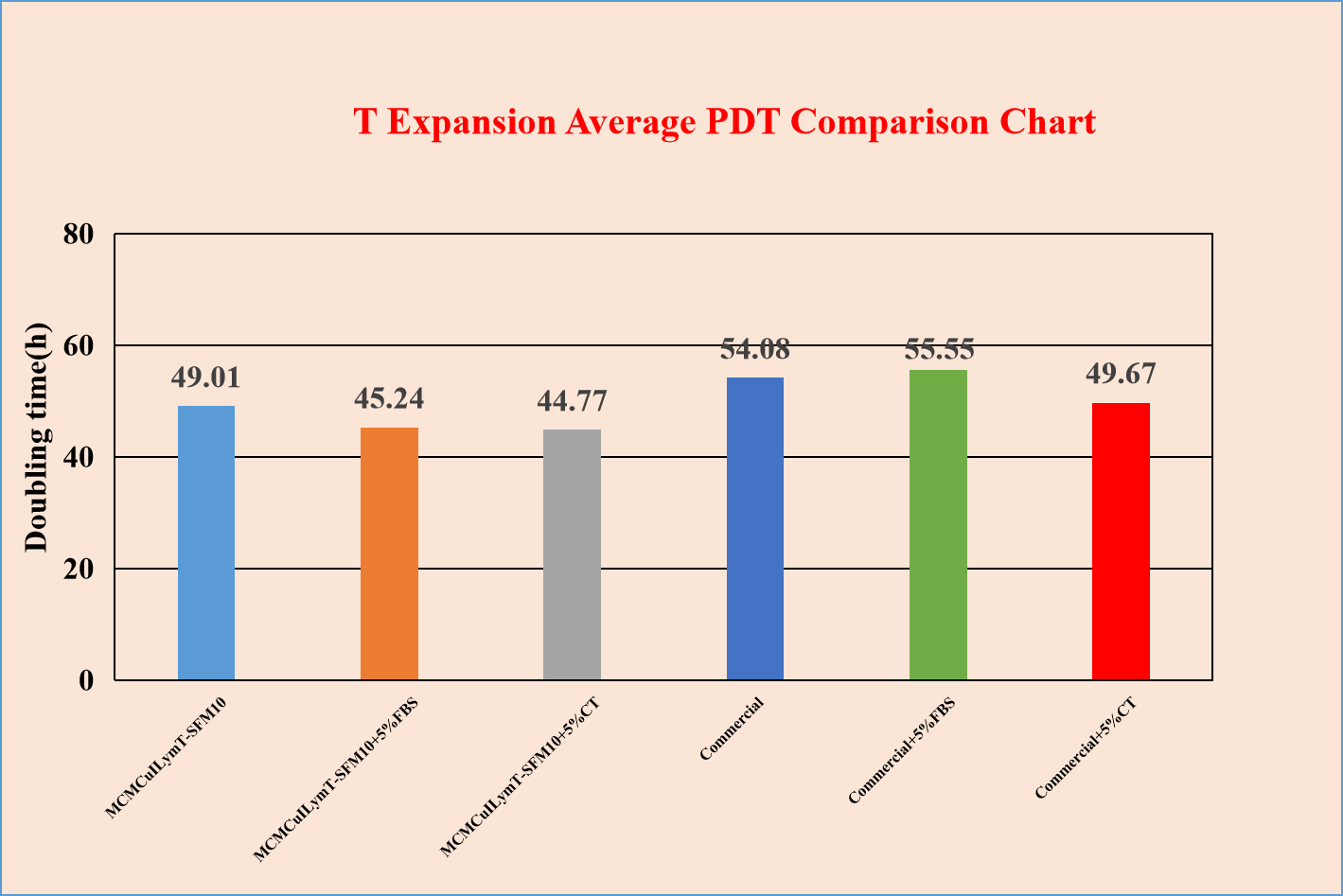
Excellent in cell proliferation, supporting T lymphocyte fast proliferation in vitro.
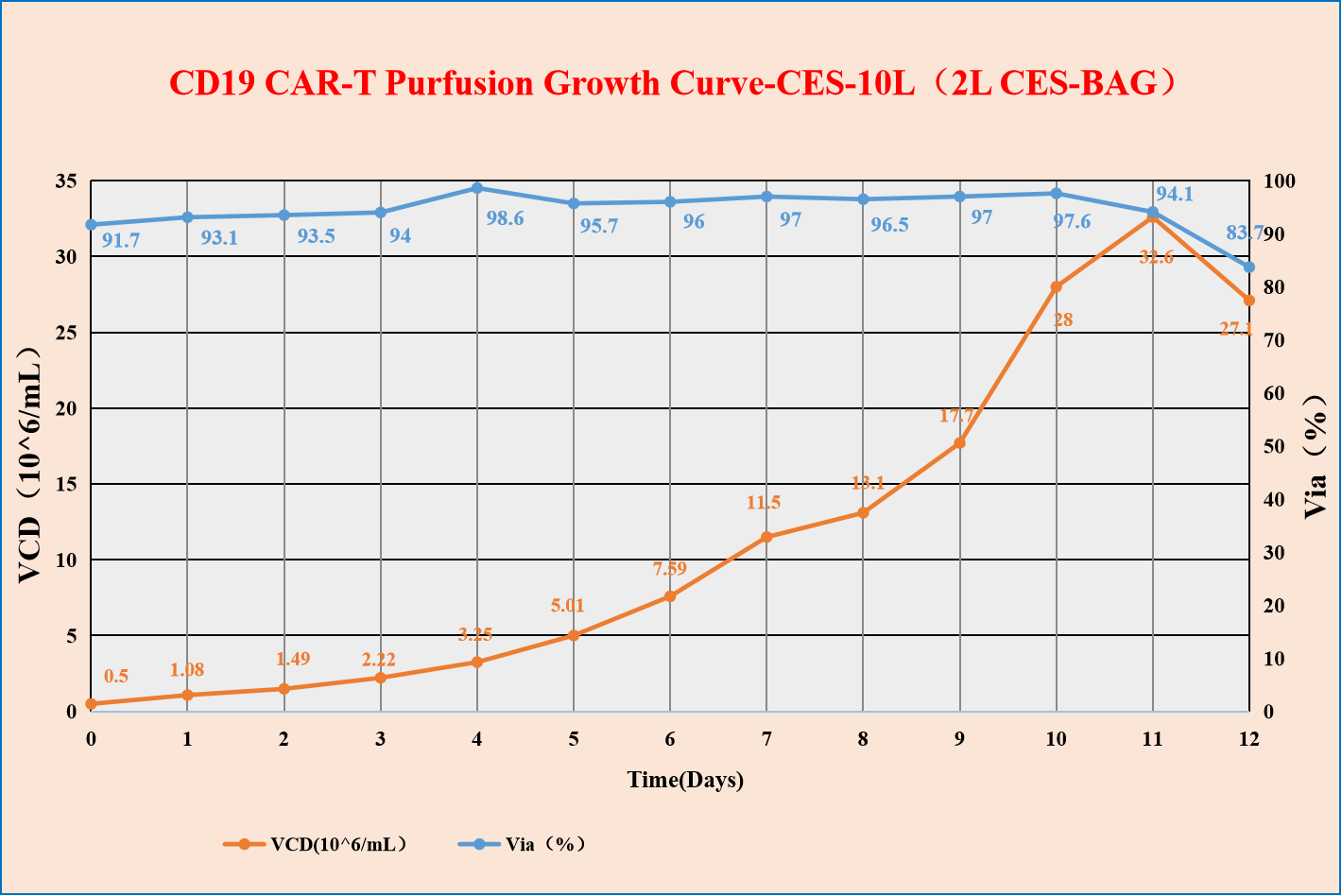
Universality, proved by multiple culture systems, such as static culture, fed-batch and perfusion culture in bioreactor
cGMP, disposable manufacturing techniques,highly stable and consistency