Application of reference technology of Tofflon HEK293 cell culture-medium family in production of different products
- 2023-03-09
- News
- 1265
1. Tofflon HEK293 cell culture medium family

| Serial number | Model number | Application | Available form | Product description |
| 1 | HEK293 CDM15 | Protein expression, AAV, AdV | Dry Powder/Liquid | Chemical composition limited, suitable for suspension culture of HEK293,HEK293F,293T and transfection of protein, AAV, AdV |
| 2 | HEK293 CDM26 | Protein expression, AAV, AdV | Dry Powder/Liquid | Suitable for suspension culture of HEK293,HEK293F,293T and transfection of protein, AAV, AdV and LV |
| 3 | HEK293 CDM30 | Protein expression, AAV, AdV | Dry Powder/Liquid | Suitable for suspension culture of HEK293,HEK293F,293T and transfection of protein, AAV, AdV and LV |
| 4 | HEK293 SFM28 | Protein expression, AAV, AdV | Liquid | No serum, suitable for 293T cell adherence maintenance and expression of transfection products |
| 5 | Supplement A (Tobitec FA) | Universal type (HEK293, CHO) | Dry Powder/Liquid | No serum, no protein and protein hydrolysate, chemical composition restriction |
| 6 | Supplement B (Tobitec FB) | Universal type (HEK293, CHO) | Dry Powder/Liquid | No serum, no protein and protein hydrolysate, chemical composition restriction |
2. Tofflon HEK293 is applied in different product transient reference process
2.1 Instantaneous reference process for the production of Dongfulon HEK293 protein
| Basal medium | Instructions | Refill | Instructions |
| HEK293 CDM15 | Contains glutamine | Supplement A (Tobitec FA) | Glutamine-free |
HEK293 CDM26 | Supplement B (Tobitec FB) | Glutamine and glucose free | |
| HEK293 CDM30 | |||
| Protein instant feed process | At about 24 and 96 h after the instantaneous transfer of plasmid, 7% FA, 0.7% FB, 4mM glutamine, glucose control to 5g/L(excluding sugar in FA) were added. | ||
| Transfection process with PEI and other chemical reagents | 1) For cells with a growth density of more than 10 e6 cells/mL in the host cell, the cell VCD was adjusted during transfection: 4.5 E6 cells/mL;DNA: 1.8mg/L; PEI:DNA=3 2) cells with host cell growth density less than 10 e6 cells/mL: adjust cell VCD during transfection: 2 E6 cells/mL;DNA: 1mg/L; PEI:DNA=3 | ||
| Other parameters suggested | Initial culture temperature 36.5℃, no cooling, pH:7.1±0.2, DO: 40%.(Recommended speed in shaker: 120rpm, amplitude: 50mm, 5-8%CO2) | ||
2.2 Tofflon HEK293 Virus production transient reference technology -- suspension system
Take the 1mL transfection system of AAV virus as an example:
| Base medium | DNA dosage | FectoVIR-AAV volume | The amount of medium used for the preparation of the complex | Supplement |
HEK293 CDM26 | 1.5 ug (1.5 ~ 2.0 ug) | 2.25 uL (1.5 ~ 4.0 ul) | 0.1 mL | Tobitec FA&Tobitec FB |
Instantaneous refill process | At about 24 and 96 h after instantaneous virus transfer, 4% FA, 0.4% FB and 4mM valley were added respectivelyAmmonia, glucose controlled to 5g/L(except sugar in FA) | |||
Carrier ratios are recommended as follows (take 1.5ug/mL DNA as an example)
| Expression vector | Molar ratio | Recommended carrier molecule Amount (Kbp) | ug DNA/mL culture volume |
| Rep/Cap plasmid | 2 | 9 | 0.95 |
| Helper plasmid | 0.5 | 11 | 0.29 |
| AAV-ITR plasmid | 1 | 5 | 0.26 |
2.3. Instantaneous Reference technology for production of HEK293 virus in Dongfulon -- Adherent system
| Basic medium | Categories | Craft |
| HEK293 SFM28 | Lentivirus Instantaneous Reference Process | 1) HEK293T adherent culture was carried out with medium (generally basic medium + serum) according to the original process, and the cell growth and fusion degree reached 80% after standing culture; 2) Viral transfection was performed using the original medium or HEK293 SFM28; 3) The serum-containing medium was removed and cleaned 4 to 6h later, and HEK293 SFM28 was added for further culture. Scandalous operation was performed 48 to 72 h after transfection. |
| Suggestions for other parameters | The initial culture temperature is 37℃, and the temperature is cooled to 32℃ after transfection |
3. Reference process for stability of Tofflon HEK293
The recommended process is as follows:
| Base medium | Stating | Refill | Instructions |
| HEK293 CDM26/30 | Already contains glutamine | Supplement A (Tobitec FA) | Glutamine-free |
| Supplement B (Tobitec FB) | Glutamine and glucose free | ||
| Feeding process | When the cell density is ≥4 e6cells/mL to start the feeding, it is recommended to add 5% per 48 hfA, 0.5% FB, 4mM glutamine (no need to add in GS system), glucose control to 6-8g/L | ||
| Other parameters recommended | Initial culture temperature 36.5℃, day5 cooling to 33℃, pH:7.1±0.2, DO: 40%. (Recommended speed in shaker: 120rpm, amplitude: 50mm, 5-8%CO2) | ||
| Remarks | 80mM Gln can be added directly to FA when mixing according to process requirements | ||
4. Tofflon HEK293 medium supporting
4.1. Provide overall solutions and application support for key reagents according to different applications and types of biological drug R&D and production:
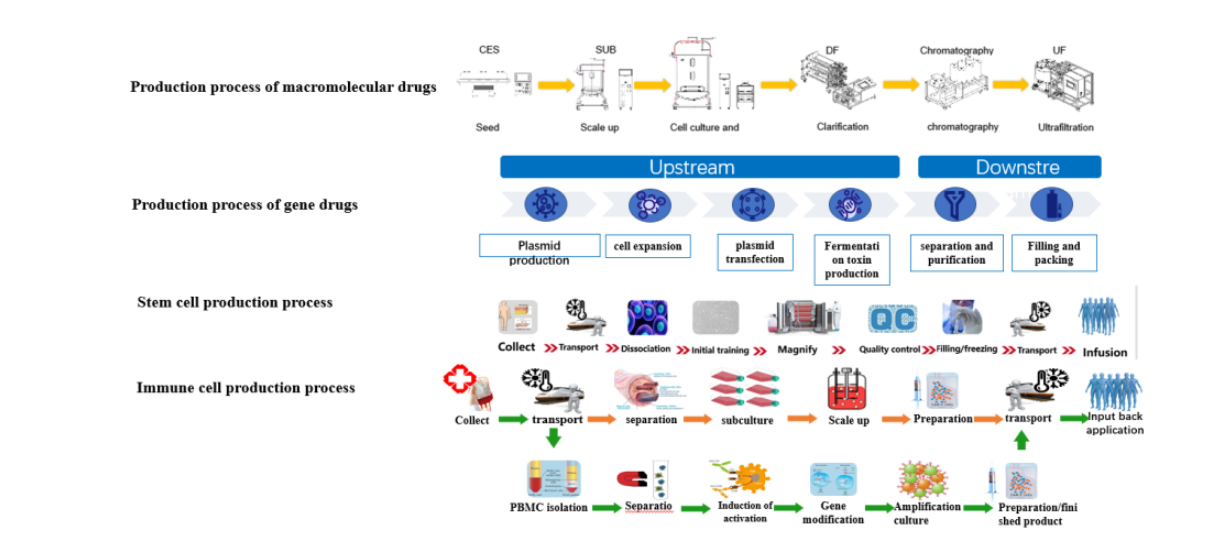
4.2. On the basis of medium application technology, combined with Tofflon's core equipment, support the scale and industrialization of biological drug projects;
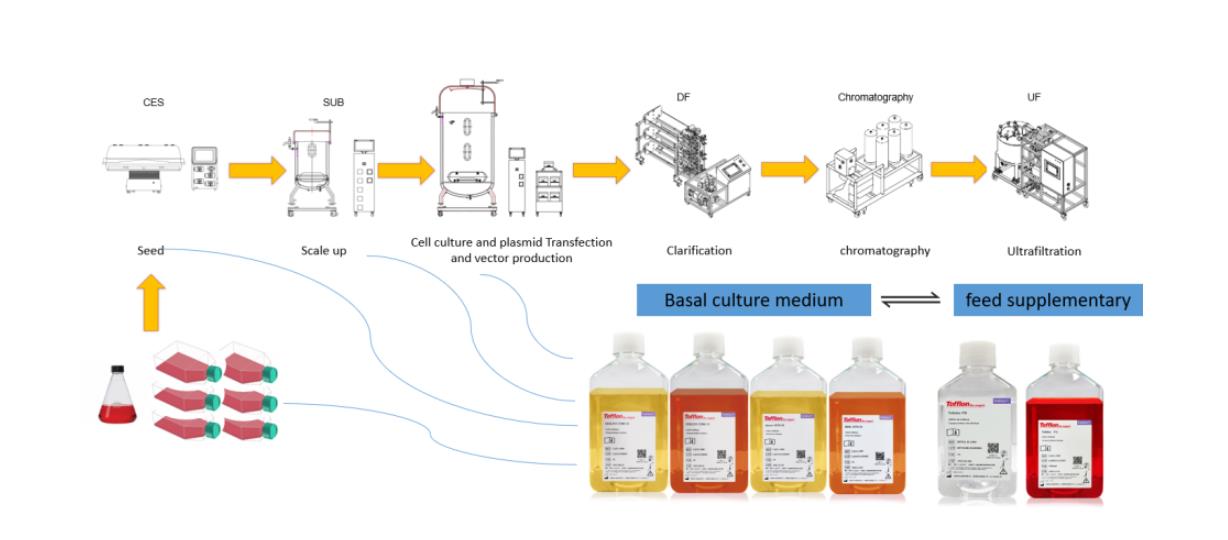
4.3. Starting from drug application and marketing, complete product documents and registration records for supporting products are provided, as well as product compliance documents and application support.




